From: National Institute of General Medical Sciences <info@nigms.nih.gov>
Date: Mon, Jul 7, 2014 at 12:13 PM
Subject: Biomedical Beat—a digest of research advances, scientist profiles, cool images and more
To: iammejtm@gmail.com
| Latest Biomedical Beat Posts | |
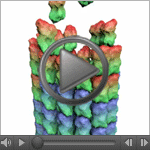 | Cool Video: How a Microtubule Builds and Deconstructs In this animation, tubulin proteins snap into place like Lego blocks to build a microtubule, part of the cell's skeleton. When construction ends, this long hollow cylinder falls to pieces from its top end. The breakdown is critical for many basic biological processes, including cell division, when rapidly shortening microtubules pull chromosomes into each daughter cell. Until recently, scientists didn't know exactly what drove microtubules to fall apart. A research team led by Eva Nogales of the Lawrence Berkeley National Laboratory and the University of California, Berkeley, now has an explanation. Using high-powered microscopy, the scientists peered into the structure of a microtubule and found how a chemical reaction puts the stacking tubulin proteins under intense strain. The only thing keeping the proteins from springing apart is the pressure from the addition of more tubulin. So when assembly stops, the microtubule deconstructs. Read more | Post a comment |
 | Carbohydrates as Bacterial Camouflage: How Our Immune System Responds When harmful strains of bacteria invade our bodies, our immune system produces antibodies that identify the intruders by the specific carbohydrate structures coating them. Some strains, however, have coatings that mimic the carbohydrate structures found on our own cells, and this disguise allows them to evade detection by antibodies. A team of scientists led by Richard Cummings of Emory University found that galectins, a class of proteins naturally produced by our bodies, can identify and kill these concealed bacteria without damaging our own mimicked cells. To make this discovery, the team used glass slides covered with more than 300 different carbohydrates extracted from the surface of bacterial cells. After testing the ability of galectins and antibodies to bind to specific carbohydrates on these slides, the researchers observed that the galectins easily detected the mammalian-like carbohydrates that the antibodies failed to recognize. Read more | Post a comment |
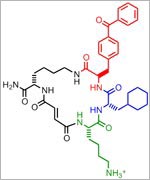 | New Compound Improves Insulin Levels in Preliminary Studies The discovery of a compound that slows the natural degradation of insulin in mice opens up a new area of investigation in the search for drugs to treat diabetes. The research team, which included David Liu and Alan Saghatelian of Harvard University, Markus Seeliger of Stony Brook University School of Medicine, and Wei-Jen Tang of the University of Chicago focused on insulin-degrading enzyme, or IDE. Using a method called DNA-templated synthesis, the scientists made 14,000 small molecules and found one that bound to the enzyme, suggesting it might modulate the enzyme's activity. Work in test tubes and in animal models confirmed this—and showed that blocking IDE activity improved insulin levels and glucose tolerance. The researchers also learned that the enzyme is misnamed: In addition to insulin, it degrades two other hormones involved in glucose regulation. Read more | Post a comment |
 | Revealing a Key Player in Cancer Metastasis Most of the more than half-a-million deaths caused by cancer each year in the United States result not from the original tumor but from the spread of cancer to new parts of the body, or metastasis. Cancer cells travel from a primary tumor using invadopodia, foot-like protrusions that break through surrounding connective tissue. Invadopodia are driven by protein filaments that repeatedly grow and disassemble. Exactly what guides this cycle was unclear, but scientists suspected a molecule called Rac1 might be involved. A new tool now sheds light on the details. Researchers led by Louis Hodgson of Albert Einstein College of Medicine developed a fluorescent biosensor that glows wherever Rac1 is active in a cell, and they used it to study highly invasive breast cancer cells taken from rodents and humans. The scientists observed invadopodia form when Rac1 activity was low and disappear when it was high. They then confirmed their findings when they shut down the gene that encodes Rac1 and saw the invadopodia remain intact indefinitely. Read more | Post a comment |
 | An Insider's Look at Life: Magnified, an Airport Exhibit of Stunning Microscopy Images Science. Art. Airports. I've never used those three words together before. But I've been doing it a lot lately while working on Life: Magnified, an exhibit of 46 striking scientific images created by scientists around the country using state-of-the-art microscopes. The show is at Washington Dulles International Airport, where more than a million travelers will see it over its 6-month run. As our director said in a recent post on another NIGMS blog, "What a great way to share the complexity and beauty of biomedical science with such a large public audience!" We've also set up an online gallery, where the colorful images can be viewed and freely downloaded for research, educational and news media purposes. Read more | Post a comment |
 | Meet Elizabeth Grice Imagine a landscape with peaks and valleys, folds and niches, cool, dry zones and hot, wet ones. Every inch is swarming with diverse communities, but there are no cities, no buildings, no fields and no forests. You've probably thought little about the inhabitants, but you see their environment every day. It's your largest organ—your skin. Elizabeth Grice, an assistant professor at the University of Pennsylvania, studies the skin microbiome to learn how and why bacteria colonize particular places on the body. Already, she's found that the bacterial communities on healthy skin are different from those on diseased skin. She hopes her work will point to ways of treating certain skin diseases, especially chronic wounds. "I like to think that I am making discoveries that will impact the way medicine is practiced," she says. Read more | Post a comment |
 | On the Trail of Drug-Defying Superbugs In the United States alone, at least 2 million people each year develop serious infections with bacteria that have become resistant to the antibiotics we use to combat them, and about 23,000 die, according to the Centers for Disease Control and Prevention. Antibiotic resistance can turn once-manageable infections into "superbug" diseases that are difficult—and sometimes impossible—to treat. Scientists funded by the National Institutes of Health are studying many aspects of antibiotic resistance, including how it spreads. Read this Inside Life Science article for just a few research examples and how the work could aid efforts to curb the emergence of resistance. Post a comment |
 | Genes control the most basic functions of the cell, including what proteins to make and when. In 2003, the Human Genome Project created a draft map of our genes, and now researchers have completed a draft map of the human proteome—the set of all our proteins. The map, which includes proteins encoded by more than 17,000 genes as well as ones from regions of the genome previously thought to be non-coding, will help advance a broad range of research into human health and disease. Post a comment |
| You received this message because you are subscribed to Biomedical Beat—a digest of short articles about research funded by the National Institute of General Medical Sciences. You also can view the articles at http://biobeat.nigms.nih.gov. | |
 | |
Questions? Contact Us SUBSCRIBER SERVICES: Manage preferences | Delete profile | Help | |
| This e-mail was sent to iammejtm@gmail.com by: National Institute of General Medical Sciences, National Institutes of Health · 45 Center Drive · Bethesda, MD 20892 · 301-496-7301 |
Jeremy Tobias Matthews

No comments:
Post a Comment